Abstract
Introduction:
Transfusion of blood and blood products is the most common medical intervention in the developed world, with someone requiring blood transfusion once every 2 seconds. With nearly 16 million units of blood products being transfused in the US annually, it is important to reflect on the transfusion trend and formulate strategies to reduce transfusion when not clinically indicated. A study was done at a community-academic center in Florence, Alabama to assess the trend of transfusion and formulate appropriate strategies to minimize transfusions that are not clinically indicated.
Methodology:
The blood management team was formed by the hospital to trend the transfusion of blood and blood products. This team consisted of hospitalists, resident physicians, nurses, blood bank personnel, and administrative personnel. The team met regularly once every month to analyze the blood transfusion trend and to calculate the rate of compliance to clinical indications for transfusion.
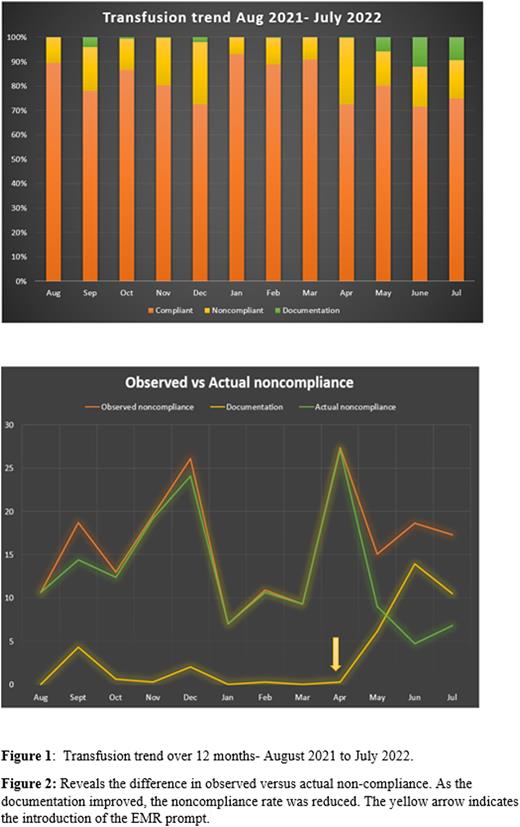
Data from August 2021 to July 2022 were analyzed to compute the rate of noncompliance. In March 2022 a prompt was introduced in the electronic medical record (EMR) system to assist with additional documentation for the ordering physician to document the indication for transfusion when the most recent hemoglobin was more than 7gm/dL.
Compliance was defined as meeting one of the clinical indications for transfusion such as hemoglobin less than 7gm/dL, active bleeding, coronary artery disease, surgery, and symptomatic anemia. Noncompliance was defined as a transfusion that did not meet these criteria.
Results
Data from August 2021 to July 2022 revealed the average number of red blood cells transfused per month to be 293 units. Indications for each of these transfusions were reviewed individually to classify them as being compliant or noncompliant with clinical indications for transfusion.
Analysis revealed an average rate of 14.4% of noncompliance from the month of August 2021 to March 2022. Following the introduction of the EMR prompt in March 2022, the documentation of the indication improved, revealing the difference between actual noncompliance and observed non-compliance. From April 2022 to July 2022, the average noncompliance rate was reduced to 10.4%
Conclusion:
The decision to transfuse often involves complex decision-making with an analysis of the risk-benefit ratio. Laboratory data needs to be correlated with the clinical scenario. Often what is considered noncompliance to clinical indication for transfusion may be a result of inadequate documentation as revealed by the data from our study. Our study serves to highlight that by utilizing simple tools on EMR, goal-directed transfusion can be achieved. In addition, the results of this study can also be generalized to other practice settings.
We intend to trend the data over subsequent months to ensure further improvement. Plans are underway to display the results of this study in various areas within the clinical workspace with easy access to physicians as a reminder for appropriate and adequate documentation while ordering blood and blood products.
Disclosures
Isaac:Pfizer: Current equity holder in private company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal